Explanation:
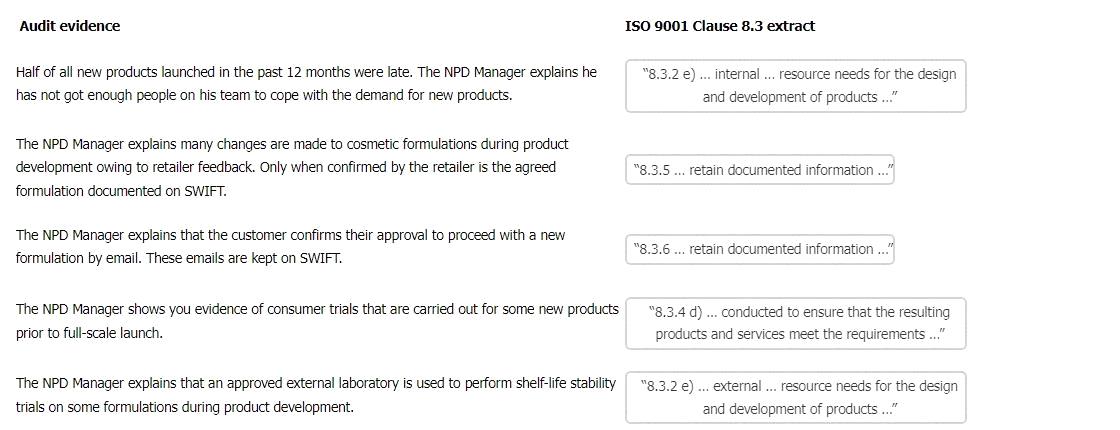
The table below shows the possible matching of the ISO 9001 Clause 8.3 extract to the audit evidence.
Table
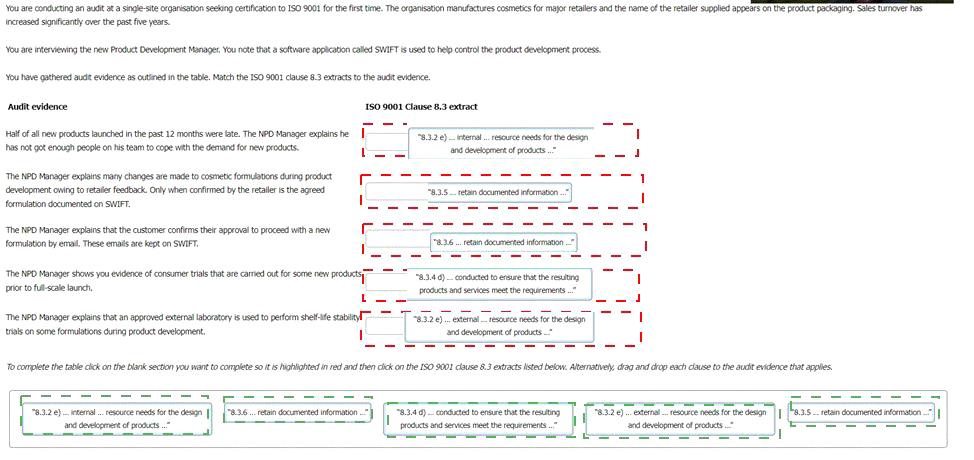
Audit evidence
ISO 9001 Clause 8.3 extract
Half of all new products launched in the past 12 months were late. The NPD Manager explains he has not got enough people on his team to cope with the demand for new products.
"8.3.2 e) ... internal ... resource needs for the design and development of products ..." The NPD Manager explains many changes are made to cosmetic formulations during product development owing to retailer feedback. Only when confirmed by the retailer is the agreed formulation documented on SWIFT.
"8.3.5 ... retain documented information ..."
The NPD Manager explains that the customer confirms their approval to proceed with a new formulation by email. These emails are kept on SWIFT.
"8.3.6 ... retain documented information ..."
The NPD Manager shows you evidence of consumer trials that are carried out for some new products prior to full-scale launch.
"8.3.4 d) ... conducted to ensure that the resulting products and services meet the requirements ..." The NPD Manager explains that an approved external laboratory is used to perform shelf-life stability trials on some formulations during product development.
"8.3.2 e) ... external ... resource needs for the design and development of products ..."